Miller S1, Liu H2, Warfvinge K3, Shi L2, Dovlatyan M2, Xu C2, Edvinsson L3.
- 1Department of Neuroscience, Amgen Inc., One Amgen Center Drive, Thousand Oaks, California 91320 and 360 Binney Street, Cambridge, MA 02142, USA. Electronic address: silkem@amgen.com.
- 2Department of Neuroscience, Amgen Inc., One Amgen Center Drive, Thousand Oaks, California 91320 and 360 Binney Street, Cambridge, MA 02142, USA.
- 3University of Lund, Institute of Clinical Sciences at Lund University Hospital, House A13, Sölvegatan, Lund 22184, Sweden.
Abstract
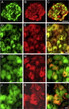
Calcitonin gene-related peptide (CGRP) is a potent vasodilator and a neuromodulator implicated in the pathophysiology of migraine. It binds to the extracellular domains of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein (RAMP) 1 that together form the CGRP receptor. Antagonist antibodies against CGRP and its binding site at the receptor are clinically effective in preventing migraine attacks. The blood-brain barrier penetration of these antagonist antibodies is limited, suggesting that a potential peripheral site of action is sufficient to prevent migraine attacks. To further understand the sites of CGRP-mediated signaling in migraine, we used immunohistochemical staining with recently developed antagonist antibodies specifically recognizing a fusion protein of the extracellular domains of RAMP1 and CLR that comprise the CGRP binding pocket at the CGRP receptor in monkey and man. We confirmed binding of the antagonist antibodies to human vascular smooth muscle cells (VSMCs) of dural meningeal arteries and neurons in the trigeminal ganglion, both of which are likely sites of action for therapeutic antibodies in migraine patients. We further used one of these antibodies for detailed mapping on cynomolgus monkey tissue and found antagonist antibody binding sites at multiple levels in the trigeminovascular system: in the dura mater VSMCs, in neurons and satellite glial cells in the trigeminal ganglion, and in neurons in the spinal trigeminal nucleus caudalis. These data reinforce and clarify our understanding of CGRP receptor localization in a pattern consistent with a role for CGRP receptors in trigeminal sensitization and migraine pathology. Copyright © 2016 IBRO. Published by Elsevier Ltd.
KEYWORDS: antibody; cynomolgus monkey; human; immunohistochemistry; migraine; trigeminal system
- PMID: 27155150
Supplement:
Since its discovery more than 35 years ago, Calcitonin-gene-related peptide (CGRP) has been extensively evaluated for its role in migraine pathophysiology (reviewed in [1]). A pioneer of this research was Lars Edvinsson at the University in Lund, Sweden. He and his co-workers found that CGRP was one of the strongest vasodilatory peptides with a primary effect on arteries, but not veins [2]. They established a possible association with migraine in a breakthrough experiment showing that release of CGRP from trigeminal nerves triggers a protective vasodilatory reflex that counteracts prolonged vasoconstriction of cerebral arteries [3]. Together with Peter Goadsby, they subsequently provided clinical data that CGRP is released into the extracerebral circulation during the headache phase of migraine [4]. These findings sparked the interest of pharmaceutical companies to develop inhibitors of CGRP signaling for the treatment of migraine.
CGRP binds to and signals through a receptor complex composed of Calcitonin like receptor (CLR) and receptor-modifying protein (RAMP)1 [5], providing a drug target to inhibit CGRP signaling. In 2004, Boehringer Ingelheim achieved clinical proof-of concept with an injectable small molecule CGRP receptor antagonist, olcegepant [6]. Merck followed with an orally available molecule, telcagepant [7], but stopped clinical development due to potential hepatotoxicity. To date, more than 10 years later, no small molecule CGRP receptor antagonist has reached approval. Unlike small molecules, antibodies have exquisite selectivity, reduced potential for drug–drug interactions, are not cleared through the liver, and their long half-life is better suited for preventative treatment. Therefore, Cen Xu and her research team at Amgen set out to develop CGRP receptor antagonist antibodies. It is very difficult to generate a receptor complex made from two components to use as an antigen. Protein engineers at Amgen designed a truncated version of the receptor composed of fragments of both RAMP1 and CLR that form the CGRP binding site (Figure 1A). They then fused these fragments together with proprietary linkers (Figure 1B) and used this construct to immunize XenoMice, transgenic mice that produce human antibodies (Figure 1C and D).
Figure 1. Cartoon illustrates the engineering of an artificial CGRP receptor binding site complex as immunogen to generate human CGRP receptor antagonist antibodies. A. Generation of extracellular domains of RAMP1 and CLR. B. Fusion of extracellular domains to proprietory linkers. C. Immunization with artificial CGRP receptor complex immunogen. D. Resulting human CGRP receptor antagonist antibody.
Erenumab (AMG 334) is one of the antibodies derived from such an immunization campaign currently in clinical trials for migraine prevention [8]. At the same time, several anti-CGRP ligand antibodies are in clinical trials by other companies. All of these antibodies have shown efficacy in preventing migraine attacks in clinical phase II studies (for review, see [9]). However, efficacy of these antibodies was not anticipated by many researchers. Migraine is viewed to originate in the central nervous system (CNS), but antibodies do not cross the blood-brain barrier in sufficient amounts to be efficacious in the CNS. Therefore, a long-standing debate about the site of action for anti-migraine efficacy was rekindled. Over many years, Lars Edvinsson and co-workers have carefully mapped the expression of CGRP and its receptors in the trigemino-vascular system from periphery to CNS (reviewed in [10]). In addition to the vasculature itself, they identified the trigeminal ganglion as another key structure within this system that is not protected by the blood-brain barrier. Peripheral fibers of trigeminal ganglion cells containing CGRP innervate the cranial vasculature, which is equipped with CGRP receptors expressed by smooth muscle cells. The CGRP containing trigeminal ganglion cells are intermingled with large neurons expressing CGRP receptors. The surrounding satellite glia cells express CGRP receptors as well. Central processes of the trigeminal ganglion cells relay inputs from the periphery to the caudal part of the spinal trigeminal nucleus and to lamina I/II of spinal C1-C3 levels and from there to higher CNS structures. This expression pattern in vasculature and trigeminal ganglion is outside the blood-brain barrier and thus provides a site for the action of CGRP or CGRP receptor antagonist antibodies.
As mentioned above, it is difficult to make antibodies against a receptor complex. Therefore, all mapping studies so far had been performed by co-localizing immunoreactivity of RAMP1 and CLR antibodies. In the current study, we asked whether CGRP receptor antagonist antibodies can be used to map the CGRP binding site at the receptor in dura vasculature and trigeminal ganglion. Since these antibodies are primate specific, we used human and cynomolgus monkey tissues. We were able to confirm previous mapping data and corroborate the evidence that dura vasculature and trigeminal ganglion may serve as primary sites of action for therapeutic CGRP receptor antagonist antibodies.
References
- Edvinsson, L., The Journey to Establish CGRP as a Migraine Target: A Retrospective View. Headache, 2015. 55(9): p. 1249-55.
- Edvinsson, L., et al., Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett, 1985. 58(2): p. 213-7.
- McCulloch, J., et al., Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A, 1986. 83(15): p. 5731-5.
- Goadsby, P.J., L. Edvinsson, and R. Ekman, Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol, 1990. 28(2): p. 183-7.
- McLatchie, L.M., et al., RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature, 1998. 393(6683): p. 333-9.
- Olesen, J., et al., Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med, 2004. 350(11): p. 1104-10.
- Ho, T.W., et al., Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet, 2008. 372(9656): p. 2115-23.
- Sun, H., et al., Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol, 2016.
- Bigal, M.E., S. Walter, and A.M. Rapoport, Therapeutic antibodies against CGRP or its receptor. Br J Clin Pharmacol, 2015. 79(6): p. 886-95.
- Edvinsson, L., CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol, 2015. 80(2): p. 193-9.
 Euro
Euro
 US Dollar
US Dollar