Verònica Casadó-Anguera*†‡1, Jordi Bonaventura†§, Estefanía Moreno*†‡, Gemma Navarro*†‡, Antoni Cortés*†‡, Sergi Ferré§ and Vicent Casadó*†‡1
*Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), 28031 Madrid, Spain.
†Department of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, 08028 Barcelona, Spain.
‡Institute of Biomedicine of the University of Barcelona (IBUB), 08028 Barcelona, Spain.
§Integrative Neurobiology Section, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, MD 21224, U.S.A.
1Correspondence may be addressed to either of these authors (email vcasadoanguera@gmail.com or vcasado@ub.edu).
Abstract
Heteromers of G-protein-coupled receptors (GPCRs) have emerged as potential novel targets for drug development. Accumulating evidence indicates that GPCRs can form homodimers and heteromers, with homodimers being the predominant species and oligomeric receptors being formed as multiples of dimers. Recently, heterotetrameric structures have been proposed for dopamine D1 receptor (D1R)–dopamine D3 receptor (D3R) and adenosine A2A receptor (A2AR)–dopamine D2 receptor (D2R) heteromers. The structural model proposed for these complexes is a heteromer constituted by two receptor homodimers. The existence of GPCR homodimers and heteromers provides a structural basis for inter-protomer allosteric mechanisms that might account for a multiplicity of unique pharmacological properties. In this review, we focus on the A2AR–D2R heterotetramer as an example of an oligomeric structure that is key in the modulation of striatal neuronal function. We also review the interfaces involved in this and other recently reported heteromers of GPCRs. Furthermore, we discuss several published studies showing the ex vivo expression of A2AR–D2R heteromers. The ability of A2AR agonists to decrease the affinity of D2R agonists has been reported and, on the basis of this interaction, A2AR antagonists have been proposed as potential drugs for the treatment of Parkinson’s disease. The heterotetrameric structure of the A2AR–D2R complex offers a novel model that can provide new clues about how to adjust the drug dosage to the expected levels of endogenous adenosine.
Supplementary
Clinical interest of GPCRs
GPCRs constitute the more extensive family of membrane proteins. They all have 7 transmembrane domains and are coupled to G proteins and arrestines that are involved in transducing extracellular signals to the intracellular environment. These extracellular signals may be light photons, hormones, neurotransmitters and neuromodulators that can be small molecules, such as the well known biogenic amines (dopamine, serotonin, norepinephrine, histamine,..), nucleosides and nucleotides (adenosine, ATP,…), lipids, odorants or bigger molecules such as peptides (CRF, orexin, oxytocin, angiotensin, vasopressin,…). In clinical medicine, GPCRs are the most important family of proteins, due to the fact that about 40% of all current marketed drugs and 20% of recently FDA approved drugs act through modulating GPCR functions. These drugs provide treatments for cancer, cardiac dysfunction, diabetes, obesity, inflammation, and pain. Moreover, they are very important for neurobiological disorders research, and it is because 90% of GPCRs are expressed in the brain and control almost all nervous system functions (Cortés et al., 2016).
Functional and pharmacological significance of dopamine and adenosine
Dopamine. Dopamine is a pleiotropic compound that acts as a neurotransmitter and a hormone. It is widely distributed in both the peripheral and the central nervous system, as well as in some peripheral non-neuronal areas including the pituitary gland, blood vessels, human adipose tissue, and the cardiovascular and renal systems. Dopamine is synthesized mainly by the nervous tissue in the dopaminergic neurons present in the substantia nigra pars compacta, in the ventral tegmental area, and in the arcuate and periventricular nucleus of the hypothalamus.
Dopaminergic neurons are involved in the control of movements, cognition, emotions, memory, attention, sleep regulation, in the pleasure and reward pathways and in the tonic inhibition of prolactin secretion by the pituitary gland (Cortés et al., 2016).
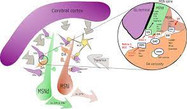
The physiological effects of dopamine are mediated by five closely related yet functionally distinct GPCRs that are divided into two major subclasses: the D1-like (D1R, D5R) and the D2-like (D2R, D3R, D4R) receptors that differ in distribution, levels of receptor expression, affinity for dopamine and in some functional properties. It is widely accepted that dopamine D1 receptor –expressed in striatum neurons- convey their information directly to the output nuclei of the basal ganglia (the direct pathway), whereas D2R-expressing neurons do so indirectly via pallidal neurons (indirect pathway) (see Figure 1). For the control of fine movements it is essential that both pathways function properly.
Adenosine. Adenosine is an endogenous purine nucleoside that acts as a homeostatic network regulator in all living systems via multiple adenosine receptors. Within cells, adenosine is involved in cellular energy and purine metabolism, but extracellular adenosine also binds to cell membrane adenosine receptors (ARs) exerting numerous physiological functions throughout the entire human body by interacting with specific ARs expressed on the surface of the target cells. There are four ARs subtypes, which either inhibit (A1R and A3R) or activate (A2AR and A2BR) adenylyl cyclase.
Adenosine plays an important role in different metabolic and pathological conditions, such as the intrarenal metabolic regulation of kidney function, asthma and hypoxia, cardiac ischemia, and it is also involved in regulating the severity of inflammation during an immune response. Moreover, it is an endogenous immunoregulator in cancer pathogenesis and has an important role controlling human brain function. Thus, adenosine plays a crucial role in neuronal excitability and synaptic/nonsynaptic transmission in the hippocampus and the basal ganglia. Because of this role, adenosine is also associated with Alzheimer’s disease, Parkinson’s disease, schizophrenia, Huntington’s disease, epilepsy, drug addiction, and sleep (Cortés et al., 2015).
In the brain, A1Rs and A2ARs are found predominantly at post-synaptic neurons in striatum, in the direct and indirect motor pathway, respectively, but they are also detected as important presynaptic neuromodulators, controlling glutamate release, in the direct motor pathway (Cortés et al., 2015).
Discovered a new protein complex that explains the lack of efficacy of adenosine antagonists
In our work “Evidence for the heterotetrameric structure of the adenosine A2A receptor-dopamine D2 receptor complex” we summarized several studies concerning the formation of heterotetrameric complexes of GPCRs and why it must be considered the minimal structural and functional unit for at least some GPCR receptors. We hypothesized that the allosteric modulations derived from the heterotetrameric complex formation may explain the lack of efficacy of antagonistic drugs, which are expected to block the action of endogenous hormones and/or neurotransmitters on a particular receptor.
This work is mainly focused on a recent study published in the Proceedings of the National Academy of Sciences of USA (PNAS) journal that has been developed by members of the University of Barcelona affiliated with the Centre for Networked Biomedical Research on Neurodegenerative Diseases (CIBERNED) led by Vicent Casadó, together with the research team led by Sergi Ferré in the National Institute on Drug Abuse (Baltimore).
New strategic therapies to fight against Parkinson’s disease
To be exact, in the PNAS study, we determined that A2ARs are associated with D2Rs in the cerebral striatum, forming tetrameric structures composed by a homodimer of A2AR together with a homodimer of D2R (Bonaventura et al., 2015). This complex becomes a new oligomeric entity that has new and different pharmachological and functional properties from those of its individual components. The former binding, produced at brain’s basal ganglia, and its function as a controller of fine movements were previously known but its particular structure was unknown. If this oligomeric structure does not work properly, severe movement disorders such as hypokinesia and hyperkinesia (movement alterations typical of pathologies such as Parkinson’s disease, schizophrenia or Huntington’s disease) appear.
To understand this fact, it needs to be known that A2AR, in non-pathological conditions, when activated by the endogenous agonist, adenosine, can decrease the affinity of D2R for its endogenous agonist, dopamine, in the A2AR-D2R heteromer. In pathological conditions, when there is a decrease on dopamine levels, such as in Parkinson’s disease, tonic levels of adenosine causes the inhibition of the indirect pathway of the movement control at the basal ganglia, movements slow down and the disequilibrium with the motor activation produced by the direct pathway generates erratic movements (see Figure 1).
On the basis of this interaction, antagonists of A2AR have been proposed as potential drugs to fight against this disease because its ability to block the inhibitory effects of adenosine on dopaminergic transduction. Istradefylline, antagonist of A2AR, has already been authorized in Japan for the treatment of patients with Parkinson’s disease. This drug counteracts the negative allosteric modulation of A2ARs activated by the endogenous levels of adenosine, potentiating the behavioral effects on D2R induced by dopamine agonists administered to the patients (L-DOPA).
The most important aspects of our research
With this study we have pointed out 3 very new remarkable aspects:
– GPCR antagonist drugs, and other natural substances such as caffeine, can produce same effects as agonists. Classically, it has been considered an agonist as a substance that binds to its receptors and is able to activate an intracellular signal. However, an antagonist it was not supposed to produce any intracellular signal being their only function to prevent agonist effects. Since the discovery of receptor oligomerization, it has been seen that agonists from one particular receptor can modulate the binding and signaling of agonists from the other receptor of the oligomer. We have also discovered that antagonists from one receptor can also modulate the binding and signaling of agonists from their partner receptor. Because of this, the potential therapeutic usage of this compounds increase.
– GPCRs do not generally appear in a monomeric form, they are and function in combination with other receptors that can be the same (homodimers) or different (heteromers) and that can modulate each other activity. We have demonstrated in our work that A2AR and D2R, both involved in the control of fine motor movement, can associate forming an entity of 4 receptors, a heterotetramer (a homodimer of A2ARs and a homodimer of D2Rs). This particular structure allows a higher complexity of ligand regulation between both types of receptors.
– Thirdly, combining these two ideas, this tetrameric structure allows to explain some weird results found in the bibliography and in our research such as the discrepancies observed when administering the same antagonist. For example, now we know that from low to moderate concentrations, caffeine can produce motor activation (as expected for an A2AR antagonist) but high doses worsen movement (as expected for an A2AR agonist). So, at high doses, an antagonist could have the same clinical outcome as an agonist. This fact has high clinical relevance because it points out that dosage with these particular antagonistic drugs is crucial for the therapeutic success.
References
Bonaventura J, Navarro G, Casadó-Anguera V, Azdad K, Rea W, Moreno E, Brugarolas M, Mallol J, Canela EI, Lluís C, Cortés A, Volkow ND, Schiffmann SN, Ferré S, Casadó V. (2015) Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer. Proc Natl Acad Sci USA 112(27):E3609-18. doi: 10.1073/pnas.1507704112.
Cortés A, Gracia E, Moreno E, Mallol J, Lluís C, Canela EI, Casadó V. (2015) Moonlighting adenosine deaminase: a target protein for drug development. Med Res Rev 35(1): 85-125. doi: 10.1002/med.21324.
Cortés A, Moreno E, Rodríguez-Ruiz M, Canela EI, Casadó V. (2016) Targeting the dopamine D3 receptor: an overview of drug design strategies. Expert Op Drug Discov 11(7):641-664. doi: 10.1080/17460441.2016.1185413.
Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sánchez-Soto M, Kumar-Barodia S, Naidu YT, Mallol J, Cortés A, Lluís C, Canela EI, Casadó V, McCormick PJ, Ferré S. (2014) Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol 86:417-429. doi: 10.1124/mol.114.093096.
Navarro G, Cordomi A, Zelman-Femiak M, Brugarolas M, Moreno E, Aguinaga D, Pérez-Benito L, Cortés A, Casadó V, Mallol J, Canela EI, Lluis C, Pardo L, García-Sáez AJ, McCormick PJ, Franco R. (2016) Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with G proteins. BMC Biology 14:26 (1-12). doi: 10.1186/s12915-016-0247-4.
Acknowledgements
This study was supported by the Government of Catalonia [grant number 2014-SGR-1236]; the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas [grant number CB06/05/0064]; the Spanish Ministerio de Economía y Competitividad [grant number SAF2014-54840-R]; and by intramural funds of the National Institute on Drug Abuse.
 Euro
Euro
 US Dollar
US Dollar